Write the molecular equation for this reaction. Which atom loses electrons in.
Solved Using Lewis Symbols Diagram The Reaction Between Chegg Com
Get the detailed answer.
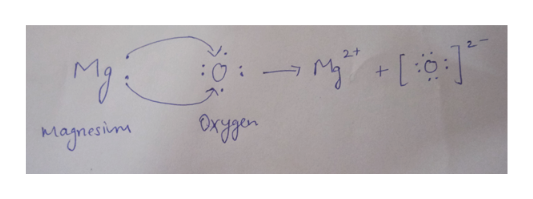
. Solution for Using Lewis symbol diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO. A Al and F b K and S c Y and O d Mg and N. B How many electrons are transferred.
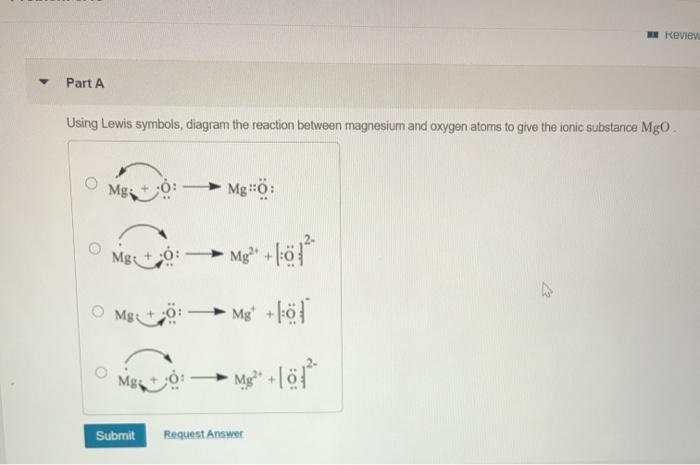
C Which atom loses electrons in the reaction. Predict the chemical formula of the ionic compound formed between the following pairs of elements. Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
C Which atom loses electrons in the reaction. Problem 15 Easy Difficulty a Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO. Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
B K and S. Solution for 815 Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO. 815 Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
The ionic bond between magnesium and oxygen is stronger. A Using Lewis symbols diagram the reaction between magnesium. Get the answer to.
A Al and F. See the answer Please explain the answer along with the solution. 815 Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance mathrmMgO. 814 Use Lewis symbols to represent the reaction that occurs CQ between Mg and Br atoms. Form and oxygen becomes O.
M g O M g 2 O 2 b. Try Numerade Free for 7 Days. Using Lewis symbol diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
This problem has been solved. 2 2- 2. You will often see electrons drawn like this in books.
The 2 Mg electrons jump to the O atom giving Mg2 and 0-2. Unlock a free month of Numerade by answering 20 questions on our new app StudyParty. A Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
Using Lewis symbol diagram the reaction between magnesium and oxygen atoms to give the ionic. Use Lewis symbols to represent the reaction that occurs between Ca and F atoms Present the transfer of electrons using aTows_ Get the answer to your homework problem. Two electrons transfer from Mg to O.
Problem Use Lewis symbols to represent the reaction that. Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO. B How many electrons are transferred.
Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgOHow many electrons are transferred. B How many electrons are transferred. Question fullscreen Expand Transcribed Image Text.
Using Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgOMgO. The one where the only two electrons from Mg are going to the oxygen with two complete pairs and two lone electrons to form Mg2 and O-2. Show transcribed image text Expert Answer 100 28 ratings.
C Y and O. 2 2 2. How many electrons are transferred.
Using lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO. B How many ele. Oxygen is in group 6 of the periodic table.
Use your knowledge of trends m electronegativity on. D Mg and N. Present the transfer of electrons using arrows 5.
Sing Lewis symbols diagram the reaction between magnesium and oxygen atoms to give the ionic sub- stance MgO. Use Lewis dot symbols in a chemical equation to show explicitly the transfer of electrons in the reaction between magnesium and chlorine atom to make the ionic compound magnesium chloride. And oxygen atoms to give the ionic substance MgO.
Magnesium metal reacts with hydrobromic acid to produce hydrogen gas and a Magnesium metal reacts with hydrobromic acid to produce hydrogen gas and a solution of magnesium bromide. Students also viewed these Organic Chemistry questions When magnesium metal is burned in air Figure 36 two products are When magnesium metal is burned in air Figure 36 two products are produced. See explanation View Answer Discussion.
An oxygen atom will gain 2 electrons to form a stable 2 - ion. 815 a Using Lewis symbols diagram the reaction between mag nesium and oxygen atoms to give the ionic substance MgO. Loses electrons in the reaction.
A magnesium atom will lose 2 electrons to form a stable 2 ion. In this example the electrons are shown as dots and crosses. So the diagram can be drawn so.
815 predict the chemical formula of the ionic compound formed between the following pairs of elements.

Lewis Structure Of Magnesium Oxide Mgo Youtube

Draw The Lewis Diagram For Mg3n2 Magnesium Nitride Youtube
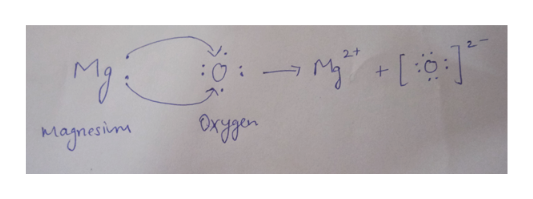
Answered 8 15 A Using Lewis Symbols Diagram Bartleby
Solved Review Part A Using Lewis Symbols Diagram The Chegg Com
0 Comments